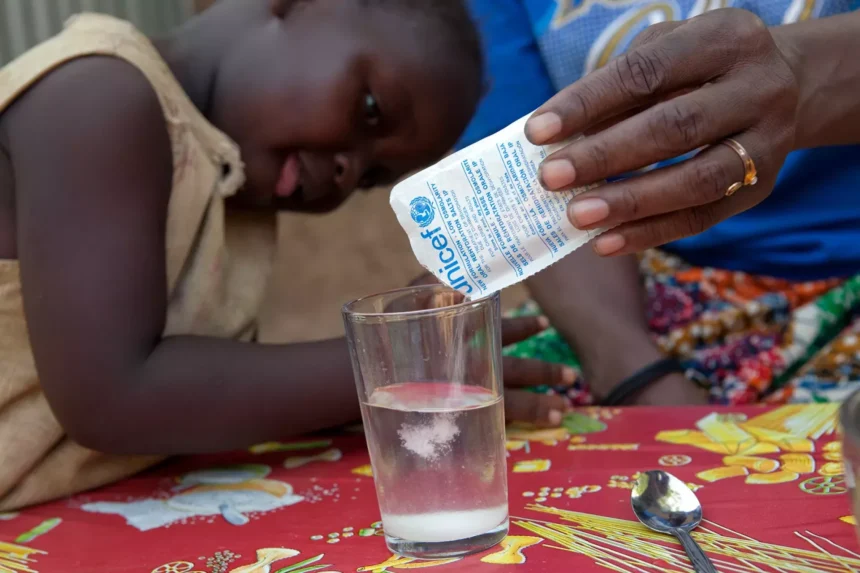
The Union government has mandated that only products approved by the World Health Organization (WHO) may be labeled as Oral Rehydration Salts (ORS), reversing earlier permissions that allowed the use of “ORS” in product names with conditional disclaimers.
In a directive issued on October 15, the Food Safety and Standards Authority of India (FSSAI) stated that the term “ORS,” whether used as a prefix or suffix in a trademark or brand name, is no longer permitted for any food product, including fruit-based, non-carbonated, or ready-to-drink options. This directive takes immediate effect.
The order rescinds permissions previously granted in July 2022 and February 2024, which had allowed companies to label their products as “ORS” provided they included disclaimers indicating the products were not WHO-recommended formulas. The FSSAI noted that such relaxations had led to “false, deceptive, ambiguous, and erroneous” labeling practices.
The regulator emphasized that the term “ORS” is associated with significant public trust, and its misuse could mislead consumers into believing that such products are capable of treating dehydration, which is not the intended medical purpose.
The new directive reaffirms an earlier statement from April 8, 2022, regarding misleading advertising and marketing of ORS substitutes, which remains effective.
The FSSAI’s decision comes amid rising concerns about the potential for consumer deception through the misuse or misbranding of ORS. ORS is a simple solution of salts and sugar combined with water, designed to treat dehydration resulting from diarrhea, heat-related illnesses, or other conditions that cause fluid loss.
Dr. Sivaranjani Santosh, a pediatrician based in Hyderabad and an advocate against mislabeling of ORS products, applauded the decision, stating, “No one can use ORS on their label unless it is a WHO-recommended formula, and no one can sell it right from today.”
Health activists and medical professionals praised Dr. Santosh for her eight-year campaign against sugar-rich drinks falsely marketed as ORS. Her efforts have been recognized as instrumental in prompting the FSSAI’s significant regulatory change.
This development highlights the importance of ensuring that labeling practices for hydration solutions are clear and in accordance with established health standards to protect consumer interests.
Tags: India bans ‘ORS’ label on non-WHO approved beverages, supplements Extract 5 SEO-friendly keywords as tags. Output only keywords, comma separated.
Hashtags: #India #bans #ORS #label #nonWHO #approved #beverages #supplements