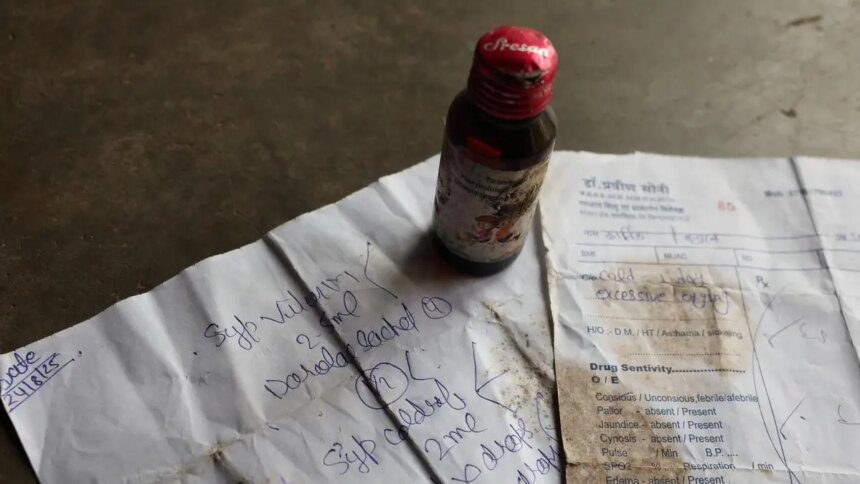
India is grappling with another tragic incident linked to contaminated cough syrups, resulting in the deaths of 25 children in Madhya Pradesh and Rajasthan. This alarming recurrence of medical negligence and ineffective drug regulation has historical roots, dating back to 1986, when 14 patients died at Mumbai’s JJ Hospital after ingesting glycerol that contained 18.5 percent diethylene glycol (DEG). Subsequent incidents included 11 child deaths in Bihar (1988), 33 in Gurgaon (1998), and 12 in Jammu & Kashmir (2020). Internationally, Indian-made cough syrups have been associated with the deaths of over 60 children in Uzbekistan and nearly 70 in Gambia.
Investigations revealed that these cough syrups were produced with industrial-grade solvents instead of pharmaceutical-grade, causing dangerous contamination with DEG, a toxic compound responsible for fatal kidney failure. In Tamil Nadu, random testing found samples of cough syrups containing 48.6 percent DEG, drastically exceeding the permissible limit of 0.1 percent. Despite previous reports and inquiries, inadequate enforcement has allowed unsafe drugs to permeate the market.
The Hathi Committee (1974-75) had proposed banning non-essential formulations, including pediatric cough syrups, due to a lack of demonstrated benefits. The World Health Organization (WHO) and the Indian Academy of Pediatrics advise against using these syrups for children under the age of four; yet, they remain readily available.
In 2023, the WHO issued alerts after Indian-made syrups were linked to mortality in Gambia. Parliamentary data indicates that 2.8 percent of drug samples tested in 2023-24 were substandard, while 0.27 percent were classified as spurious or adulterated. A 2024 investigation by the Central Drugs Standard Control Organization (CDSCO) revealed that over 100 pharmaceutical units producing cough syrups for export had failed quality assessments. These statistics underline serious deficiencies within India’s drug regulatory framework. Recommendations from the Mashelkar Committee to appoint one drug inspector per 50 factories and another for every 200 retail outlets have not been implemented. Regulatory enforcement remains severely lacking.
Accountability for maintaining drug safety falls on pharmaceutical companies, the CDSCO, and state regulators. However, irrational, over-the-counter cough syrups with dubious formulations still flood the marketplace. Recently, medical associations have raised concerns over the arrest of a doctor in Madhya Pradesh, shedding light on broader issues of accountability.
Victims, predominantly from marginalized communities, often find themselves without recourse due to delayed actions, the absence of a drug recall policy, and a lack of compensation frameworks for drug-related injuries. India continues to struggle with inadequate mechanisms for addressing adverse drug reactions and holding companies and officials responsible.
Public trust in the regulatory system has further diminished following reports that more than 30 pharmaceutical companies acquired electoral bonds totaling over ₹900 crore, raising significant conflict of interest concerns.
The Way Forward
For India to address these critical issues effectively, it must take decisive action. This includes banning irrational drug formulations, filling vacant regulatory positions, and strengthening drug testing infrastructures. Implementing modern quality management systems is vital for regulatory authorities to restore credibility and safeguard lives. Recent advisories promoting rational prescribing are a step in the right direction, yet they are insufficient. Civil society organizations are calling for an outright ban on irrational cough syrups, accountability for officials, and the establishment of a judicial commission to investigate these tragic events.
This situation transcends mere regulatory failure; it represents a national disgrace. Both Central and State governments must shoulder collective responsibility. As seen in the aftermath of the 1937 U.S. drug tragedy that led to the establishment of the U.S. Food and Drug Administration, India must learn from its historical missteps and empower regulators to prioritize patient safety over profit.
(The writer is National Convenor, Jan Swasthya Abhiyan India. Views are personal)
Published on October 20, 2025